Terephthalic acid (TPA) 100-21-0
Price: contact
Terephthalic acid is an organic compound with the chemical formula C6H4(COOH)2. The colorless solid is a commodity chemical primarily used as a precursor to the polyester PET used to make clothing and plastic bottles. Millions of tons are produced every year. It is one of three isomeric phthalic acids.
| describe | Terephthalic acid is an organic compound with the chemical formula C6H4 ( COOH) 2 . The colorless solid is a commodity chemical primarily used as a precursor to the polyester PET used to make clothing and plastic bottles. Millions of tons are produced every year. It is one of three isomeric phthalic acids. |
| chemical properties | Terephthalic acid is a white crystalline solid. It is easily soluble in alkaline solution, slightly soluble in hot ethanol, and insoluble in water, ether, glacial acetic acid and chloroform. Therefore, until around 1970, most of the crude terephthalic acid was converted into dimethyl ester for purification. It sublimates when heated. |
| history | Terephthalic acid came to attention around 1940 from the work of Winfield and Dickson in England. Early work by Carothers and colleagues in the United States established the feasibility of producing high molecular weight linear polyesters by reacting diacids with diols, but they used aliphatic diacids and diols. These made polyesters are not suitable for spinning into fibers. Winfield and Dickson discovered that symmetric aromatic diacids produced high-melting, crystalline, and fiber-forming materials; polyethylene terephthalate (PET) has since become the most produced synthetic fiber. |
| application | 1,4-phthalic acid is mainly used in the production of polyethylene terephthalate. It also produces the plasticizer dioctyl phthalate (DOTP) and polyester plasticizers. 1,4-phthalic acid undergoes a condensation reaction with polyols to prepare polyester plasticizers with diethylene glycol, triethylene glycol, glycerol, propylene glycol, butylene glycol, etc. |
| use | Terephthalic acid (TPA) is a benzene polycarboxylic acid with potential antihemorrhagic properties. It is a high-tonnage chemical used extensively in the production of synthetic materials, particularly polyester fibers (polyethylene terephthalate). |
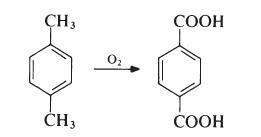
| Prepare | The main commercial route to terephthalic acid suitable for direct preparation of polyethylene terephthalate starts from p-xylene:
Paraxylene is primarily obtained from petroleum sources and is a product of the fractionation of reformed naphtha. Oxidation takes place in the liquid phase. Typically, air is passed into a solution of p-xylene acetic acid at about 200°C. In the presence of a catalyst system containing cobalt and manganese salts and a source of bromide ions, the pressure is 2MPa (20 atmospheres). The terephthalic acid produced contains only small amounts of impurities (mainly p-carboxybenzaldehyde) and can be easily removed. The acid is dissolved in water at about 2500 e and 5 MPa (50 atmospheres) and treated with hydrogen (converting the aldehyde to p-toluic acid). The solution was then cooled to 100°C? Pure terephthalic acid crystals. |
| definition | ChEBI: Terephthalic acid is a phthalic acid with carboxyl groups in the 1 and 4 positions. One of three possible isomers of phthalic acid, the others being phthalic acid and isophthalic acid. It is the conjugate acid of terephthalate (1-). |
| synthesis | Benzoic acid, phthalic acid and other benzenecarboxylic acids in the form of alkali metal salts constitute the starting material. In the first step, the alkali metal salt (usually potassium) is converted to terephthalate when heated to temperatures above 350 °C (662 °F). A dry potassium salt (benzoic acid or phthalic acid or isophthalic acid) is heated in anhydrous form in the presence of an inert atmosphere (CO2) and a catalyst (usually cadmium benzoate, cadmium phthalate) to about 420 °C (788 °F), oxides or carbonates). Corresponding zinc compounds have also been used as catalysts. In the next step, the reaction product was dissolved in water and terephthalic acid was precipitated with dilute sulfuric acid. The yield of terephthalic acid ranges from 95% to 98%. |
| application | In fact, the worldwide supply of terephthalic acid and dimethyl terephthalate is consumed as a precursor to polyethylene terephthalate (PET). World production in 1970 was approximately 1.75 million tons. By 2006, the global demand for purified terephthalic acid (PTA) had exceeded 30 million tons. The demand for terephthalic acid is small but still important in the production of polybutylene terephthalate and several other engineering polymers. |
| production method | Terephthalic acid is produced by the oxidation of paraxylene with oxygen in the air: the reaction proceeds through the paratoluic acid intermediate, which is then oxidized to terephthalic acid. In p-toluic acid, the electron-withdrawing carboxylic acid group deactivates the methyl group, making it only one-tenth as reactive as xylene itself, making the second oxidation more difficult. This commercial process uses acetic acid as solvent and a catalyst consisting of cobalt and manganese salts and a bromide promoter. |
| synthetic references | Chemistry Letters, 15, p. 299, Journal of the American Chemical Society 1986 , 82, p. 14. 2876, 1960 DOI: 10.1021/ja01496a051 Journal of Organic Chemistry, 44, p. 4727, 1979 DOI: 10.1021/jo00393a063 |
| general instructions | White powder. |
| air and water reaction | Insoluble in water. |
| reactive profile | Terephthalic acid is a carboxylic acid. If there is a base that accepts hydrogen ions, terephthalic acid releases hydrogen ions. This "neutralization" generates large amounts of heat and produces water and salt. Carboxylic acids are insoluble in water, but even "insoluble" carboxylic acids can absorb enough water from the air and dissolve sufficiently in terephthalic acid to corrode or dissolve iron, steel, and aluminum parts and containers. May react with cyanide salts to form gaseous hydrogen cyanide. Reacts with cyanide solution to release gaseous hydrogen cyanide. Reactions with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrogen compounds and sulfides may produce flammable and/or toxic gases and heat. Reacts with sulfites, nitrites, thiosulfates (generating H2S and SO3), dithionite (SO2) to produce flammable and/or toxic gases and heat. Reaction with carbonates and bicarbonates produces a harmless gas (carbon dioxide), but heat is still produced. It can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. May initiate polymerization; may catalyze (increase) the rate of chemical reactions. |
| fire hazard | There are no flash point data for terephthalic acid. Terephthalic acid may be flammable. |
| Flammability and explosiveness | non-flammable |
| Security overview | The intravenous and intraperitoneal routes are moderately toxic. Mildly toxic if swallowed. Irritating to eyes and may explode during preparation. When heated and decomposed, acrid and irritating fumes are released. |
| potential contact | TPA is primarily used in the production of polyethylene terephthalate polymer, which is used to make polyester fibers and films. A mass-produced chemical in the United States. |
| Purification method | The acid is purified by the sodium salt which, after crystallization from water, is reconverted to the acid by acidification with a mineral acid. The solid was filtered off, washed with water and dried in vacuo. S-benzylisothiouronium salt has m 204o (from aq. EtOH). [Beilstein 9 IV 3301. ] |
| Incompatibility | Flammable; dust may form explosive mixtures with air. Carboxyl compounds react with all bases, inorganic and organic (i.e. amines), releasing large amounts of heat, water and potentially harmful salts. With arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, thiols, nitrogen compounds and sulfides (releases heat, toxic and possibly flammable gases), thio Sulfates and dithionites (releasing hydrogen sulfate and sulfur oxides) are incompatible. Incompatible with oxidizing agents (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fire or explosion. Keep away from alkaline substances, strong bases, strong acids, oxo acids, epoxides. |
| waste disposal | The material is dissolved or mixed with a flammable solvent and burned in a chemical incinerator equipped with an afterburner and scrubber. All federal, state and local environmental regulations must be followed. |
| Upstream and downstream product information of terephthalic acid |
| raw material | Acetic acid --> p-xylene -- > bromide -- > cobalt acetate --> manganese triacetate dihydrate --> paraldehyde --> manganese (II) acetate --> 1,1,2, 2-Tetrabromoethane --> 4- formylbenzoic acid--> p -Toluic acid-->terephthalaldehyde -- > 1,4-dibromobenzene --> [4-(hydroxymethyl ) cyclohexyl] methanol |
| Preparation products | Pigment Red 122 --> Triethylene glycol dimethacrylate --> Unsaturated polyester resin --> Dimethyl terephthalate --> Polybutylene terephthalate --> Paraphenylene Diformyl chloride --> Luminol --> Tassah silk fabric finishing agent --> Polyethylene terephthalate --> Dioctyl terephthalate --> Liquid crystal heterocyclic polymer-- > Auxiliary chrome tanning agent --> 1,4-bis(trifluoromethyl)-benzene --> 1,4-bis(pentafluorophenyl)phthalate |
Product categories
7-Hydroxybenzofuran (furan phenol) 4790-81-2
2,2-Oxydiethanol
Expandable microspheres
PP&engineering plastic lightweight functional masterbatch
N,N'-(4,4'-methylenediphenyl)bismaleimide 13676-54-5
2-Ethyl-2-(hydroxymethyl)propane-1,3-diol nonanoic acid
Xanthate
Xanthate
Didecyl phthalate 84-77-5
Dinonyl phthalate 84-76-4
Methacrylate 922-67-8
Trichloroisocyanuric acid 87-90-1
Trichloropolyamine Urate
N-undecane
4,4'-Bismaleimidediphenylmethane 13676-54-5
Isooctyl acrylate 103-11-7
Trihydroxymethylpropane
1-cyanoguanidine (dicyandiamide)
Sodium cyclamate (cyclamate) 139-05-9
Phthalic anhydride 85-44-9
Vinyl acetate 108-05-4
Diethylene glycol 111-46-6
Acetaminophen 103-90-2
Isophthalic acid 121-91-5
Didecyl phthalate 84-77-5
Methacrylate 922-67-8
Terephthalic acid (TPA) 100-21-0
Butyl acrylate 141-32-2
Methacrylate 79-41-4
Methyl methacrylate 80-62-6
Hydroxyethyl methacrylate hema 868-77-9
Dimethyl adipate 627-93-0
Dimethyl succinate 106-65-0
Dbe dibasic ester 95481-62-2
Dimethyl glutarate 1119-40-0
1,5-pentanediol 111-29-5
N-dodecane 112-40-3
Allyl glycidyl ether 106-92-3
Pyromellitic dianhydride PMDA 89-32-7
Propylene glycol methyl ether acetate PMA
Propylene glycol methyl ether PM
N-heptane 142-82-5
Tetramethylammonium hydroxide 25% 2.38% 75-59-2
Methyl acetate 79-20-9
Tetrachlorethylene 127-18-4
Trichlorethylene 79-01-6
Xylene 1330-20-7
Cyclohexanone
Absolute ethanol 64-17-5
Butyl acetate 123-86-4
Acetone 67-64-1
Butanone 78-93-3
Toluene 108-88-3
N-propyl bromide npb
Monofluorodichloroethane (HCFC-141b)
TF-B5 lead-free cleaning agent
PP treatment agent
DMP-30 Editor
Polyamide resin 63428-84-2
Isobornyl methacrylate IBOMA 7534-94-3
Dipentaerythritol hexaacrylate DPHA 29570-58-9
Stain remover oil stain remover oil stain remover oil
Film cleaner, film water, film cleaner, PCB potion
Trimethylolpropane trimethacrylate TMPTMA 3290-92-4
Trimethylolpropane triacrylate TMPTA
Scratch card ink matching thinner for screen printing
Pentaerythritol Triacrylate 3524-68-3
Methaclean
Epoxy resin 593 curing agent
Film cleaner
Isoparaffin D60 solvent oil
Different ice 98-82-8
Tripropylene glycol diacrylate TPGDA 42978-66-5
Dipropylene glycol diacrylate DPGDA
Carbon dodecyl to tetradecyl glycidyl ether 68609-97-2
HCFC-141B High Purity Cleaning Agent High Purity Sanaifu Original
1,6-Hexanediol diglycidyl ether 16096-31-4
1,4-Butanediol diglycidyl ether 2425-79-8
Flame retardant cleaning agent (non-flammable cleaning agent)
Epoxy reactive diluent 692
Neopentyl glycol diglycidyl ether 17557-23-2
Trimethylolpropane triglycidyl ether 30499-70-8
Butyl glycidyl ether
Polypropylene glycol diglycidyl ether 26142-30-3
Polyethylene glycol diglycidyl ether 39443-66-8
Paint stripper
Industrial alcohol
New solvent---a substitute for butanone
Butyl glycidyl ether (BGE) 2426-08-6
Hydrofluoroether 347 - 406-78-0
Ethyl acid ester [4]
Phenyl glycidyl ether 122-60-1
Dipropylene glycol diacrylate DPGDA
Isopropyl alcohol 67-63-0
TA-55B ink cleaning agent
Flux cleaning agent
Lead-free no-clean TF-9000 series
Traditional no-wash TF-800T series
TF-2000-3 environmentally friendly cleaning agent
GB38508-2020 cleaning agent
High pressure anti-wear hydraulic oil HM46
Halogen-free environmentally friendly cleaning agent
Circuit board cleaning agent
Synthetic high-speed electric discharge machining oil EDM-2
Stamping oil stain cleaner
Environmentally friendly flash point-free cleaning agent
Hydrocarbon ultrasonic cleaning agent
718 net washing water is
ENEOS cleaning agent Japan NSclean 100 hydrocarbon cleaning agent
High flash point hydrocarbon cleaning agent (safe and non-flammable) XHC-280 complies with VOC
Hydrocarbon cleaning agent
Lead-free washing water
Aluminum cleaning agent
Copyright © 2021. All rights reserved Thiết kế web iHappy.