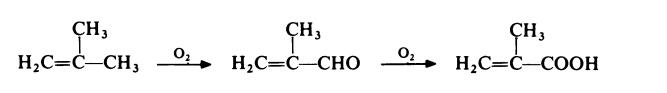Product
Methacrylate 79-41-4
Price: contact
Methacrylic acid, abbreviated as MAA, is an organic compound. This colorless, viscous liquid is a carboxylic acid with a pungent, unpleasant odour. Soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, notably methyl methacrylate (MMA) and poly(methyl methacrylate) (PMMA). Methacrylates are used in a variety of ways, most notably in the manufacture of polymers traded under the trade names Plexiglass and Perspex. Small amounts of MAA are naturally present in Roman Chamomile Oil.
| describe | Methacrylic acid, abbreviated as MAA, is an organic compound. This colorless, viscous liquid is a carboxylic acid with a pungent and unpleasant odor. Soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, notably methyl methacrylate (MMA) and poly(methyl methacrylate) (PMMA). Methacrylates are used in a variety of ways, most notably in the manufacture of polymers with the trade names Plexiglas and Plexiglas. Small amounts of MAA are naturally present in Roman Chamomile Oil. |
| chemical properties | Methacrylic acid is a colorless, moderately volatile, corrosive liquid with a strong pungent odour. It was first prepared in 1865 from ethyl methacrylate obtained by dehydration of ethyl α-hydroxyisobutyrate. |
| use | Methacrylic acid is used to make methacrylate resins and plastics. It is used as a monomer for bulk resins and polymers, organic synthesis. Many polymers are based on esters of acids such as methyl, butyl or isobutyl. Methacrylic acid and methacrylates are used to prepare a variety of polymers [→ polyacrylamides and polyacrylic acids, → polymethacrylates]. Polymethyl methacrylate is the main polymer in this category, providing clear, tough plastics in sheet form for use in glass, signs, displays and lighting panels. |
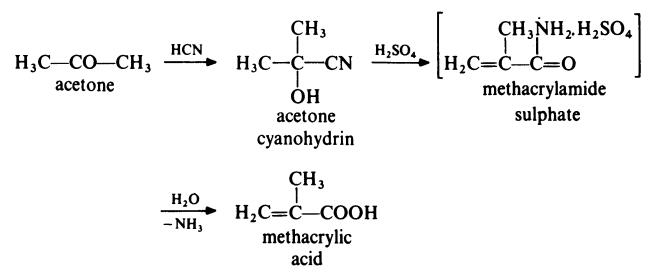
| production method | The most common method for the synthesis of methacrylic acid is the hydrolysis of methacrylamide sulfate, obtained from acetone cyanide. Methyl methacrylate can be prepared directly in a similar manner by adding methanol in the final reaction step. In the manufacture of methacrylic acid, methacrylamide sulfate is reacted with water under conditions similar to those used to form the ester. The reactor effluent separated into two phases. The upper organic layer was distilled to provide pure methacrylic acid. The lower layer is stripped to recover the dilute aqueous methacrylic acid solution, which is recycled to the hydrolysis reactor. The spent acid stream is disposed of as in ester manufacture. |
| Prepare | The most common route to prepare methacrylic acid is from acetone as follows:
In a typical process, acetone is treated with hydrogen cyanide at 140°C with ammonia as the catalyst. The generated acetone cyanide is treated with concentrated sulfuric acid at 100°C to generate sulfate methacrylamide. This intermediate is not isolated but converted directly to methacrylic acid by treatment with water at about 90°C. A competitive route currently in commercial operation involves the two-stage oxidation of isobutene with air. The reaction proceeds with methacrolein:
|
| definition | ChEBI: Methacrylic acid is an α,β-unsaturated monocarboxylic acid, which is acrylic acid in which the hydrogen at the 2-position of acrylic acid is replaced by a methyl group. It is functionally related to acrylic. It is the conjugate acid of methacrylate. |
| general instructions | Methacrylic acid is a clear, colorless liquid (or low-melting solid) with a pungent odor. Corrodes metals and tissues. Flash point 170°F. Melting point 61°F. Exothermic polymerization may occur if heated or contaminated. If polymerization occurs within a container, the container may rupture violently. Less dense than water. Vapor is heavier than air. Used to make plastics. |
| air and water reaction | Dissolved in water. |
| reactive profile | Methacrylic acid reacts with strong oxidizing agents. Storage hazard: Vigorously exothermic polymerization may occur spontaneously, resulting in explosion, especially at low concentrations of inhibitors or stabilizers [Anonymous, CISHC Chem. Safety Summary, 1979, p. 50, p. 11 . 34; Bond, J., Loss Prevention. Bulletin, 1991, 101 pages. 1]. |
| health hazard | Methacrylic acid is a highly corrosive liquid. Contact with eyes may cause blindness. Skin contact may cause burns. No inhalation toxicity was observed in rats. Exposure to its vapors may cause mild to moderate skin and eye irritation. The rabbit transdermal LD50 value is 500 mg/kg. |
| fire hazard | Flammable liquid; flash point (open cup) 76°C (170°F); vapor pressure at 20°C (68°F) Methacrylic acid readily polymerizes. The reaction is exothermic. The rate of reaction increases when heated and closed containers may rupture violently. Trace amounts of hydroquinone and hydroquinone monomethyl ether can inhibit polymerization (Aldrich 2006). Acids can be safely stored at temperatures below their melting point. |
| Security overview | Poisoning via the intraperitoneal route. Moderately toxic by ingestion and skin contact. Corrosive to skin, eyes, and mucous membranes. Report mutation data. Flammable when exposed to heat, flame or oxidizing agents. Storage hazard; exothermic polymerization may occur spontaneously. When extinguishing fire, please use alcohol foam, spray, mist, dry powder. When heated and decomposed, acrid and irritating fumes are released. |
| potential contact | Methacrylic acid is used in the preparation of methacrylic acid esters and carboxylated polymers; in the production of materials or their alkyl esters, as a monomer or comonomer for synthetic resins for the production of plastic sheets, moldings and fibers. |
| Carcinogenicity | Methacrylic acid was considered by the IARC working group but no monograph was prepared due to insufficient carcinogenicity data. The IUCLID database reports a dermal application study (dose not specified) in which mice were treated 3 times a week for 4 months and then observed for life. No excess dermal tumors were observed. |
| Shipping | UN2531 Methacrylic acid, stable, hazard class: 8; label: 8 - Corrosive material. |
| Purification method | The aqueous methacrylic acid solution (90%) was saturated with NaCl (to remove most of the water), then the organic phase was dried over CaCl2 and distilled under vacuum. Polymerization inhibitors should be added to the distillate, including 0.25% p-methoxyphenol, 0.1% hydroquinone or 0.05% N,N'-diphenyl-p-phenylenediamine. [Berstein 2 IV 1518. ] |
| Incompatibility | Vapors may form explosive mixtures with air. Reducing agent; incompatible with oxidizing agents (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fire or explosion. Aqueous solutions are strongly acidic: incompatible with strong acids; caustics, ammonia, amines, isocyanates, alkylene oxides; epichlorohydrin. Polymerizes readily when heated above 59 F/15 C, or in the presence of light, oxidants such as peroxides; or presents a fire or explosion hazard in the presence of traces of hydrochloric acid. Etches metal. Note: Typically contains 100 ppm monomethyl ether hydroquinone (150-76-5) as an inhibitor to prevent polymerization |
| waste disposal | The material is dissolved or mixed with a flammable solvent and burned in a chemical incinerator equipped with an afterburner and scrubber. All federal, state and local environmental regulations must be followed. |
| Methacrylic acid upstream and downstream product information |
Product categories
7-Hydroxybenzofuran (furan phenol) 4790-81-2
2,2-Oxydiethanol
Expandable microspheres
PP&engineering plastic lightweight functional masterbatch
N,N'-(4,4'-methylenediphenyl)bismaleimide 13676-54-5
2-Ethyl-2-(hydroxymethyl)propane-1,3-diol nonanoic acid
Xanthate
Xanthate
Didecyl phthalate 84-77-5
Dinonyl phthalate 84-76-4
Methacrylate 922-67-8
Trichloroisocyanuric acid 87-90-1
Trichloropolyamine Urate
N-undecane
4,4'-Bismaleimidediphenylmethane 13676-54-5
Isooctyl acrylate 103-11-7
Trihydroxymethylpropane
1-cyanoguanidine (dicyandiamide)
Sodium cyclamate (cyclamate) 139-05-9
Phthalic anhydride 85-44-9
Vinyl acetate 108-05-4
Diethylene glycol 111-46-6
Acetaminophen 103-90-2
Isophthalic acid 121-91-5
Didecyl phthalate 84-77-5
Methacrylate 922-67-8
Terephthalic acid (TPA) 100-21-0
Butyl acrylate 141-32-2
Methacrylate 79-41-4
Methyl methacrylate 80-62-6
Hydroxyethyl methacrylate hema 868-77-9
Dimethyl adipate 627-93-0
Dimethyl succinate 106-65-0
Dbe dibasic ester 95481-62-2
Dimethyl glutarate 1119-40-0
1,5-pentanediol 111-29-5
N-dodecane 112-40-3
Allyl glycidyl ether 106-92-3
Pyromellitic dianhydride PMDA 89-32-7
Propylene glycol methyl ether acetate PMA
Propylene glycol methyl ether PM
N-heptane 142-82-5
Tetramethylammonium hydroxide 25% 2.38% 75-59-2
Methyl acetate 79-20-9
Tetrachlorethylene 127-18-4
Trichlorethylene 79-01-6
Xylene 1330-20-7
Cyclohexanone
Absolute ethanol 64-17-5
Butyl acetate 123-86-4
Acetone 67-64-1
Butanone 78-93-3
Toluene 108-88-3
N-propyl bromide npb
Monofluorodichloroethane (HCFC-141b)
TF-B5 lead-free cleaning agent
PP treatment agent
DMP-30 Editor
Polyamide resin 63428-84-2
Isobornyl methacrylate IBOMA 7534-94-3
Dipentaerythritol hexaacrylate DPHA 29570-58-9
Stain remover oil stain remover oil stain remover oil
Film cleaner, film water, film cleaner, PCB potion
Trimethylolpropane trimethacrylate TMPTMA 3290-92-4
Trimethylolpropane triacrylate TMPTA
Scratch card ink matching thinner for screen printing
Pentaerythritol Triacrylate 3524-68-3
Methaclean
Epoxy resin 593 curing agent
Film cleaner
Isoparaffin D60 solvent oil
Different ice 98-82-8
Tripropylene glycol diacrylate TPGDA 42978-66-5
Dipropylene glycol diacrylate DPGDA
Carbon dodecyl to tetradecyl glycidyl ether 68609-97-2
HCFC-141B High Purity Cleaning Agent High Purity Sanaifu Original
1,6-Hexanediol diglycidyl ether 16096-31-4
1,4-Butanediol diglycidyl ether 2425-79-8
Flame retardant cleaning agent (non-flammable cleaning agent)
Epoxy reactive diluent 692
Neopentyl glycol diglycidyl ether 17557-23-2
Trimethylolpropane triglycidyl ether 30499-70-8
Butyl glycidyl ether
Polypropylene glycol diglycidyl ether 26142-30-3
Polyethylene glycol diglycidyl ether 39443-66-8
Paint stripper
Industrial alcohol
New solvent---a substitute for butanone
Butyl glycidyl ether (BGE) 2426-08-6
Hydrofluoroether 347 - 406-78-0
Ethyl acid ester [4]
Phenyl glycidyl ether 122-60-1
Dipropylene glycol diacrylate DPGDA
Isopropyl alcohol 67-63-0
TA-55B ink cleaning agent
Flux cleaning agent
Lead-free no-clean TF-9000 series
Traditional no-wash TF-800T series
TF-2000-3 environmentally friendly cleaning agent
GB38508-2020 cleaning agent
High pressure anti-wear hydraulic oil HM46
Halogen-free environmentally friendly cleaning agent
Circuit board cleaning agent
Synthetic high-speed electric discharge machining oil EDM-2
Stamping oil stain cleaner
Environmentally friendly flash point-free cleaning agent
Hydrocarbon ultrasonic cleaning agent
718 net washing water is
ENEOS cleaning agent Japan NSclean 100 hydrocarbon cleaning agent
High flash point hydrocarbon cleaning agent (safe and non-flammable) XHC-280 complies with VOC
Hydrocarbon cleaning agent
Lead-free washing water
Aluminum cleaning agent
Copyright © 2021. All rights reserved Thiết kế web iHappy.