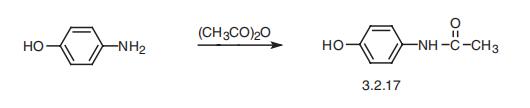Product
Acetaminophen 103-90-2
The chemical name of acetaminophen is N-(4-hydroxyphenyl)acetamide, and its trade name is paracetamol, which belongs to the acetanilide class of antipyretic and analgesics. It was first synthesized by Morse in 1878 and first clinically used by Von Mering in 1893. It became an over-the-counter drug in the United States in 1955, and production began in my country in the late 1950s. Acetaminophen appears as white crystals or crystalline powder, with a melting point of 168°C to 172°C. It is odorless and has a slightly bitter taste. It is easily soluble in hot water or ethanol, soluble in acetone, and almost insoluble in cold water and petroleum ether. It is stable below 45℃, but will be hydrolyzed to p-aminophenol when exposed to humid air and further oxidized.
| Antipyretic and analgesic | The chemical name of acetaminophen is N-(4-hydroxyphenyl)acetamide, and its trade name is paracetamol, which belongs to the acetanilide class of antipyretic and analgesics. It was first synthesized by Morse in 1878 and first clinically used by Von Mering in 1893. It became an over-the-counter drug in the United States in 1955, and production began in my country in the late 1950s. Acetaminophen appears as white crystals or crystalline powder, with a melting point of 168°C to 172°C. It is odorless and has a slightly bitter taste. It is easily soluble in hot water or ethanol, soluble in acetone, and almost insoluble in cold water and petroleum ether. It is stable below 45℃, but will be hydrolyzed to p-aminophenol when exposed to humid air and further oxidized. Varying in color from pink to brown to black, acetaminophen exerts its antipyretic effects by inhibiting the synthesis of thermoregulatory prostaglandins in the hypothalamus, with an antipyretic effect similar in intensity to that of aspirin. On the other hand, acetaminophen can produce analgesic effects by inhibiting the synthesis of prostaglandins in the central nervous system and blocking the impulses of noxious nerve endings, but it is weaker than aspirin. Compared with aspirin, acetaminophen has the advantages of less irritation and less allergic reactions. Its antipyretic and analgesic effects are similar to those of phenacetin. Since many countries have restricted or banned the use of phenacetin, the use of acetaminophen has increased. Clinically, it is mainly used for fever and headache caused by colds, and to relieve mild to moderate pain in joints. Pain, muscle pain, neuralgia, migraine, dysmenorrhea, cancer pain, postoperative analgesia, etc. It is suitable for patients who are allergic to aspirin, intolerant to aspirin or not suitable for taking aspirin, such as patients with chickenpox, hemophilia and other bleeding diseases (including patients receiving anticoagulant therapy), and patients with mild peptic ulcer and gastritis. In addition, it can also be used in the synthesis of benzoates, as asymmetric synthesis intermediates, photographic chemicals and stabilizers for hydrogen peroxide. Hemophilia and other bleeding disorders (including patients receiving anticoagulant therapy), as well as patients with mild peptic ulcer and gastritis. In addition, it can also be used in the synthesis of benzoates, as asymmetric synthesis intermediates, photographic chemicals and stabilizers for hydrogen peroxide. Hemophilia and other bleeding disorders (including patients receiving anticoagulant therapy), as well as patients with mild peptic ulcer and gastritis. In addition, it can also be used in the synthesis of benzoates, as asymmetric synthesis intermediates, photographic chemicals and stabilizers for hydrogen peroxide. |
| chemical properties | Prismatic crystals were obtained from ethanol. Melting point 169-171℃, relative density 1.293 (21/4℃). Easily soluble in ethanol, acetone and hot water, insoluble in water, insoluble in petroleum ether and benzene. Odorless, bitter taste. The pH value of saturated aqueous solution is 5.5-6.5. |
| Pharmacological effects | Acetaminophen is used as an antipyretic and analgesic. It exerts an antipyretic effect by inhibiting cyclooxygenase to mediate peripheral vasodilation and perspiration, and selectively inhibits the synthesis of thermoregulating prostaglandins in the hypothalamus. The intensity of its antipyretic effect is similar to that of aspirin. As a peripheral analgesic, it inhibits the synthesis and release of prostaglandins and increases the pain threshold to produce analgesic effects. However, its effect is weaker than aspirin and is only effective for mild to moderate pain. No obvious anti-inflammatory effect. |
| Pharmacokinetics | Oral absorption is rapid and complete, with the peak time being 0.5 to 2 hours. The plasma protein binding rate is 25% to 50%. This product is evenly distributed in the body, 90% to 95% is metabolized in the liver, and is mainly excreted from the kidneys with glucuronic acid. About 3% is excreted in the urine as unchanged form within 24 hours. Its half-life (t1/2) is 1 to 4h (average 2h). T1/2 is not affected in renal insufficiency, but t1/2 may increase in patients with liver insufficiency, neonates, or elderly patients, and t1/2 may decrease in children. It is secreted in breast milk. |
| Preparation | 1. Use nitrobenzene as raw material in the presence of concentrated sulfuric acid and cetylmethyl ammonium chloride, and use Pd/C as the catalyst to convert nitrobenzene into p-aminophenol through catalytic hydrogenation. Acetaminophen was synthesized by acetaminophen via one-step acylation method without separation, with a yield of 64.3%. The reaction is as follows: 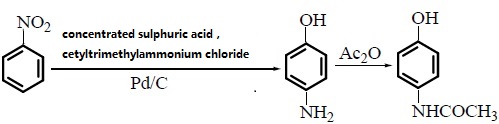 2. Use p-nitrophenol as the raw material, paracetamol as the raw material, Pd/C as the catalyst, and synthesize acetaminophen through one-step hydrogenation and acylation. The best solvent is acetic acid, its dosage is 2 to 5 times that of p-nitrophenol, and the yield of acetaminophen can reach 95%. When using Pd-La/C as catalyst, the yield can reach 97%. The reaction is as follows: 3. Use para-aminophenol as raw material , para-aminophenol and acetic anhydride as raw material, zinc powder as antioxidant, activated carbon as decolorizing agent, dilute acetic acid as reaction medium, and use microwave radiation technology to synthesize acetaminophen. The yield Up to 81. 2%. The reaction is as follows: 4. Using p-hydroxyacetophenone as raw material, first oximate p-hydroxyacetophenone, and then rearrange it through the Beckmann method to obtain acetaminophen. This method oximates hydroxyacetophenone to obtain 4-hydroxyacetophenone oxime with a yield of 93.5%. Then, using Hβ molecular sieve as the catalyst and acetone as the solvent, acetaminophen was obtained through rearrangement with a yield of 81.2%. The rearrangement reaction uses acetone as the solvent and Al-MCM-41 molecular sieve as the catalyst. The highest yield occurs when the phosphoric acid content in the catalyst is 30%. The reaction is as follows: 5. Using phenol as raw material , acetaminophen is synthesized through acetylation, Fries rearrangement, oxime and Beckmann rearrangement. The yields were 82%, 68.6%, and 50.5%, respectively. The reaction is as follows: 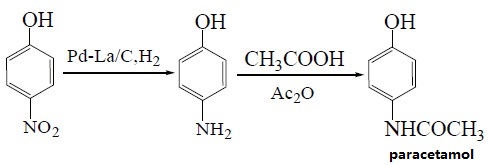 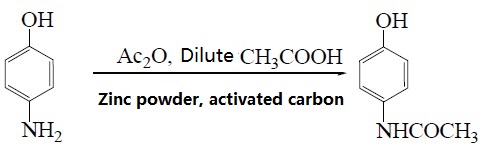 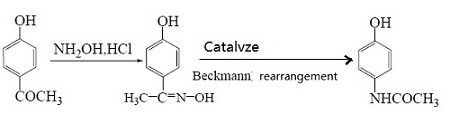 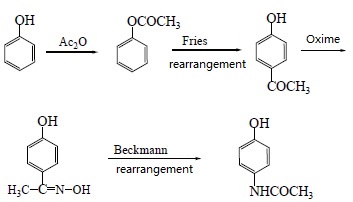 |
| Dosage | Uses This product is an antipyretic and analgesic drug, and its international nonproprietary name is Paracetamol. It is the most common non-anti-inflammatory analgesic and antipyretic drug, without anti-inflammatory and anti-rheumatic effects. Its antipyretic effect is similar to that of aspirin, but its analgesic effect is weaker. It is the best acetanilide drug. This product is particularly suitable for patients who cannot take carboxylic acid drugs. For colds, toothache. Acetaminophen is also used as an organic synthesis intermediate, hydrogen peroxide stabilizer, and photographic chemicals. Dosage 1. Oral administration (1) Paracetamol tablets or paracetamol capsules: 300 to 600 mg each time for adults, 3 to 4 times a day, as needed. The daily dosage should not be more than 2g. Antipyretic treatment generally lasts no more than 3 days, and analgesic treatment lasts no more than 10 days. Children take 10-15mg/kg every 4-6 hours. For children under 12 years old, the dosage should not exceed 5 times a day, with a maximum course of 5 days. This product is not suitable for long-term use. 2. Dispersible tablets: Disperse the tablets with warm water when taking. The usual dosage for children is 10 to 15 mg/kg every 4 to 6 hours. For children under 12 years old, the dosage should not exceed 5 times a day, with a maximum course of 5 days. Reduce dosage for children under 3 years old. |
| Application in specific diseases | Osteoarthritis:
|
| Adverse reactions | 1. Allergic reactions: Except for occasional allergic reactions such as rash and urticaria, the side effects of one treatment are less and mild. Rarely, methemoglobinemia may occur. 2. Liver and kidney damage: Long-term heavy use can lead to liver and kidney damage, thrombocytopenia, and even jaundice, oliguria, acute severe hepatitis, and severe cases can lead to coma and death. High doses may cause nausea, vomiting, stomach pain, stomach cramps, diarrhea, anorexia, sweating, etc. 3. Children under 3 years old have immature liver and kidney functions, poor detoxification and excretion functions, and should try to avoid using this product. In addition, patients with liver and kidney insufficiency and pregnant women should use it with caution. Long-term drug addicts should check their renal function and blood picture regularly. |
| taboo | People who are allergic to this product and those with severe hepatic and renal insufficiency are contraindicated. |
| notes |
|
| medicine interactions |
|
| Administrative point of care |
|
| usage | Organic synthesis intermediates, hydrogen peroxide stabilizers, photographic chemicals, non-anti-inflammatory analgesic antipyretics. |
| Production | 由对氨基苯酚乙酰化而得。 方法一:将对氨基苯酚加入稀醋酸中,加入冰醋酸,升温至150℃反应7h,加入醋酐反应2h,检查终点,验收后冷却至25℃,摇匀过滤,加水至无醋酸味,干燥得粗品。 方法二:对氨基苯酚、乙酸和含酸50%以上的工业酸一起蒸馏,蒸馏稀酸的速度为1小时内总馏出液的1/10,检查对氨基苯酚残留量小于2.5内温升至130℃时取样检测氨基苯酚%,加入稀酸(含量50%以上),冷却结晶。摇匀,过滤后,先用少量稀酸洗涤,然后用大量水至滤液近无色,得粗品。方法1的收率为90%,而方法2的收率为90-95%。精制方法:将水加热至近沸时加入粗品。升温至完全溶解,加入用水浸泡的活性炭,用稀乙酸调节至pH=4.2-4.6,煮沸10min。压滤机、滤液中加入少量亚硫酸氢钠。冷却至20℃以下,析出晶体。摇匀后过滤,洗涤,干燥,得活性成分扑热息痛成品。 其他生产方法有: (1)对硝基苯酚在乙酸中用锌还原,同时乙酰化得到对乙酰氨基酚。 (2)将对羟基苯乙酮生成的腙放入含硫酸的酸性溶液中,然后加入亚硝酸钠,反转得到对乙酰氨基酚。 |
| 描述 | 对乙酰氨基酚与所描述的非甾体类抗炎药的不同之处在于它缺乏抗炎和抗风湿特性。最近的研究表明,对乙酰氨基酚与阿司匹林一样,可以抑制大脑中环氧合酶的作用,甚至比阿司匹林更强。另一方面,乙酰胺奥芬的镇痛作用机制尚不完全清楚,因为它对外周环氧合酶的作用较差。 |
| 描述 | 对乙酰氨基酚是一种镇痛和解热化合物。与许多同时抑制 COX-1 和 COX-2 的非甾体抗炎药不同,早期研究表明对乙酰氨基酚对这两种亚型的抑制剂效果较差。然而,尽管在 1,000 mg 高剂量下,它确实能在体外抑制人血液中 83% 的 COX-2 和 56% 的 COX-1 ,IC 为50值分别为 25.8 和 113.7 μM。对乙酰氨基酚通过酶促和非酶促转化为几种反应性代谢物,这些代谢物会导致不良或间接影响,包括肝损伤。在中毒剂量下,对乙酰氨基酚代谢物 N-乙酰基-4-苯醌亚胺 (NAPQI;) 会耗尽肝脏中的谷胱甘肽储备,导致 NAPQI 积聚并随后导致肝细胞坏死。当以 250 mg/kg 的剂量给药时,对乙酰氨基酚会降低小鼠体内的谷胱甘肽水平并降低谷胱甘肽过氧化物酶活性,并诱导原代小鼠肝细胞中的铁死亡,这种作用可以被铁死亡抑制剂 ferostatin-1 阻断。对乙酰氨基酚在动物模型中具有镇痛和解热作用。 |
| 化学性质 | 白色固体 |
| 鼻祖 | Trigesic,施贵宝,美国,1950 |
| 用途 | 镇痛;退烧药 |
| 用途 | 抗感染剂 |
| 用途 | 液体闪烁计数中的分散剂 |
| 用途 | 制造偶氮染料、照相化学品。 |
| 用途 | 对乙酰氨基酚广泛用作镇痛和退烧剂。对乙酰氨基酚设计用于中度镇痛。它也像阿司匹林一样有效,用于镇痛头痛(从弱到中度疼痛)、肌痛、关节痛、慢性疼痛、肿瘤和术后疼痛等。 |
| 定义 | ChEBI:扑热息痛是酚类的一员,它是 4-氨基苯酚,其中与氨基相连的一个氢已被乙酰基取代。它具有环氧合酶 2 抑制剂、环氧合酶 1 抑制剂、非麻醉性镇痛药、解热剂、非甾体抗炎药、环氧合酶 3 抑制剂、异生素、环境污染物、人血清代谢物的作用,一种肝毒性剂、铁死亡诱导剂和老年保护剂。它是酚类的成员,也是乙酰胺的成员。它在功能上与 4-氨基苯酚相关。 |
| 适应症 | 对乙酰氨基酚(泰诺)是一种有效的解热镇痛药,在治疗剂量下耐受性良好。其抗炎活性较弱;因此,它不能用于治疗类风湿性关节炎和其他炎症性疾病。 |
| 制造流程 | 将硝基苯在硫酸溶液中电解还原得到的约250ml反应混合物经测定含有约23克帕氨基苯酚,在60℃至65℃的温度下用碳酸钙中和至pH为4.5 。滤出形成的硫酸钙沉淀,用约65℃的热水洗涤沉淀,然后合并滤液和洗涤水。然后用每份25ml的苯萃取该溶液两次,并且对于每份存在的对氨基苯酚,用0.5重量份的活性炭处理水相,并将后者滤出。活性炭通过用热稀碱处理、然后用热稀酸洗涤来再生,并至少重复使用三次。 然后在40℃下向获得的滤液中加入约0.2克连二亚硫酸钠或亚硫酸钠和15.0克在约27克乙酸酐中的无水乙酸钠。将形成的反应混合物在搅拌下冷却至8℃至10℃并在该温度下保持60分钟。获得约27克N-乙酰基-帕氨基苯酚的结晶沉淀物,其熔点为169-171℃。这相当于85%的产率。 可以使用氢氧化钙、氢氧化钡、氯化钡或形成不溶性硫酸盐的其他碱土金属盐或氢氧化物来代替碳酸钙作为中和剂。 |
| 品牌 | 乙酰苯 (G & W);婴儿发烧(阿特维斯);Injectapap(Ortho-McNeil);Neopap(综合医学);泰诺(麦克尼尔);Anacin;Crocin。 |
| 治疗功能 | 镇痛、解热 |
| 世界卫生组织 (WHO) | 扑热息痛是一种广泛使用的镇痛和解热药,已知在服用过量的情况下会导致肝损伤,通常会导致致命的后果。在推荐剂量下,不会发生这种风险。扑热息痛被列入世界卫生组织基本药物标准清单。 |
| 合成参考文献 | 有机化学杂志,27,p。1092, 1962 DOI: 10.1021/jo01050a543 四面体字母,22,第 1092 页 1257, 1981 DOI: 10.1016/S0040-4039(01)90289-8 |
| 一般说明 | 无臭白色结晶固体。苦味道。pH(饱和水溶液)约6。 |
| 空气和水反应 | 微溶于水。 |
| 反应性概况 | 对乙酰氨基酚对光敏感。与强氧化剂不相容。 |
| 火灾危险 | 没有对乙酰氨基酚的闪点数据;然而,对乙酰氨基酚可能是可燃的。 |
| 可燃性和爆炸性 | 非易燃 |
| 生物活性 | 环氧合酶抑制剂;可能对 COX-3 具有选择性(犬 COX-3、鼠 COX-1 和 COX-2 的 IC 50 值分别为 460、> 1000 和 > 1000 μM)。广泛用作镇痛解热剂。 |
| 作用机制 | 对乙酰氨基酚的作用机制尚不清楚,但它可能以与 NSAID 类似的方式发挥作用,抑制环加氧酶 COX-1 和 COX-2,以减少 COX-1 和 2 活性所需的苯氧基自由基形成和前列腺素的合成。它对低浓度的过氧化物酶和花生四烯酸具有选择性抑制前列腺素合成的作用,但在较高浓度下效果有限,因此抗炎作用有限。与阿片类药物不同,扑热息痛没有明确的内源性结合位点。在某些情况下,它可能对 COX-2 抑制表现出优先作用。越来越多的证据表明扑热息痛具有中枢镇痛作用。还发现它可以阻止细胞转录浓度下前列腺素的产生, |
| 药代动力学 | 口服后,扑热息痛从小肠迅速吸收;30-60 分钟后达到峰值血浆浓度。它也可以直肠和静脉注射(作为扑热息痛或前药丙扑热息痛)。具有良好的口服生物利用度(70%~90%);直肠吸收变化较大(生物利用度~50%–80%),达到峰值血浆浓度的时间较长。血浆半衰期约为2-3小时。 扑热息痛通过肝微粒体酶主要代谢为葡萄糖醛酸、硫酸盐和半胱氨酸结合物。这些代谢物均不具有药理活性。最小量的代谢物 N-乙酰基-氨基-苯醌亚胺通常由细胞色素 P450 介导的羟基化产生。这种反应性有毒代谢物通过与肝脏谷胱甘肽结合而变得无害,然后以硫醇衍生物的形式通过肾脏排泄。使用较大剂量的扑热息痛时,反应性代谢物的形成速率超过谷胱甘肽结合物,并且反应性代谢物与肝细胞大分子结合,导致细胞死亡和潜在的致命性肝衰竭。诱导细胞色素 P450 酶的药物(例如巴比妥类药物或卡马西平)会增加这种代谢物的形成。 |
| 临床应用 | 对乙酰氨基酚呈弱酸性(pKa = 9.51),由对氨基苯酚乙酰化合成。它与血浆蛋白的结合较弱(18-25%)。对乙酰氨基酚可用作解热/镇痛药,特别是对阿司匹林过敏或敏感的个体。它不具有抗炎活性,但可以对多种关节炎和肌肉骨骼疾病产生镇痛作用。它有多种剂型,包括栓剂、片剂、胶囊、颗粒剂和溶液。成人的常用剂量为每 4 至 6 小时 325 至 650 毫克。由于潜在的肝毒性问题,不建议长期治疗的剂量超过 2.6 克/天。对乙酰氨基酚与阿司匹林不同,在水溶液中稳定,使得液体制剂易于使用, |
| 合成 | 对乙酰氨基酚,即对乙酰氨基苯酚(3.2.80),是通过对氨基苯酚与乙酸酐反应合成的[76,77]。
|
| 环境命运 | 虽然摄入剂量的对乙酰氨基酚的大部分已被解毒,但极小部分通过细胞色素 P450 混合功能氧化酶途径代谢为高反应性的 n-乙酰基-对苯醌亚胺 (NAPQI)。有毒中间体NAPQI通常被内源性谷胱甘肽解毒为半胱氨酸和硫醇酸结合物并通过尿液排出。最近的研究表明,肝脏 P450、CYP2E1 以及较小程度上的 CYP1A2 负责将对乙酰氨基酚转化为 NAPQI。对乙酰氨基酚过量时,NAPQI 量增加并耗尽内源性谷胱甘肽储备。时程研究表明,反应性 NAPQI 的共价结合和随后的毒性仅在细胞谷胱甘肽储存量减少 70% 或更多时才会发生。小鼠中毒剂量后 15 分钟即可观察到线粒体功能障碍和损伤,表明这可能是细胞坏死的关键。NAPQI被认为与肝细胞中的关键细胞大分子共价结合并导致细胞死亡。最近的蛋白质组学研究已经鉴定出至少 20 种已知蛋白质被反应性对乙酰氨基酚代谢物共价修饰。由此产生的对乙酰氨基酚-半胱氨酸 (APAP-CYS) 蛋白加合物可以通过高压液相色谱结合电化学检测 (HPLC-EC) 进行定量。肝细胞死亡导致肝坏死和炎症,导致出现与肝功能衰竭一致的临床和实验室结果。 |
| 代谢途径 | 对乙酰氨基酚 (APAP) 由小鼠代谢,在尿液中鉴定出九种代谢物。主要代谢物是APAP-葡萄糖醛酸和3-半胱氨酰-APAP。S-(2,5-二羟基苯基)半胱氨酸和S-(2,5-二羟基苯基)-N-乙酰半胱氨酸的氢醌代谢物源自APAP的苯醌代谢物。 |
| 代谢 | 对乙酰氨基酚主要通过结合反应在胃肠道中进行快速首过代谢,其中 O-硫酸盐结合物是儿童的主要代谢物,O-葡萄糖醛酸苷是成人的主要代谢物。对乙酰氨基酚和非那西丁的一个次要但重要的产物是 CYP2E1 和 CYP3A4 产生的 N-羟基酰胺。 |
| 贮存 | 储存于 RT |
| 纯化方法 | 从水或乙醇中重结晶扑热息痛。3,5-二硝基苯甲酰胺络合物从热水中得到橙色晶体,m 171.5o。[贝尔斯坦 13 H 460、13 I 159、13 II 243、13 III 1056、13 IV 1091。] |
| 对乙酰氨基酚 上下游产品信息 |
| 原料 | 盐酸-->乙酸-->乙酐-->铁-->亚硫酸氢钠-->活性炭-->氧化镁-->焦亚硫酸钠--> 4-氨基苯酚--> 4'-羟基苯乙酮- ->二苯甲酮腙-->硝基苯-->乙酰胺,N-(4-氯-2-羟基苯基)- |
| 制剂产品 | Cefoperazone -->Phenacetin -- > Obendazole -- >Diphenethyl peptide--> Ethyl 5-benzyloxyindole-2-carboxylate --> β-(4-( Acetylamino)phenoxy)propionic acid --> 4-isothiocyanate-4'-nitrodiphenyl ether --> N-(4-(epoxymethoxy)-1,2-epoxypropane --> 4-(Acetamido)phenyl chloroacetate --> Ethyl 4-(Acetamido)phenoxyacetate --> 6-Acetamidobenzo-4-one --> 6-Fluoro- 4-chromanone --> 4-amino-3-nitrophenol --> 3,4-dimethoxyphenol --> 4-pentyloxyaniline --> acid green 62 --> mordant green 2 |