Diethylene glycol 111-46-6
Price: contact
Chemical Properties Diethylene glycol is a clear, colorless, odorless, stable oily liquid. It is also slightly tacky, non-corrosive and non-volatile. Due to its ether and alcohol groups, diethylene glycol exhibits the chemistry of primary alcohols and ethers. It has a much higher boiling point than ethylene glycol and is more solvent-sensitive. Second B
| chemical properties | Diethylene glycol is a clear, colorless, odorless, stable oily liquid. It is also slightly tacky, non-corrosive and non-volatile. Due to its ether and alcohol groups, diethylene glycol exhibits the chemistry of primary alcohols and ethers. It has a much higher boiling point than ethylene glycol and is more solvent-sensitive. Diethylene glycol is miscible with water, ether, lower aliphatic alcohols, aldehydes and ketones, and partially soluble in benzene, carbon tetrachloride, monobenzene, o-dichlorobenzene and toluene. It dissolves many dyes, resins, oils, nitrocellulose and many organic substances. Due to its solvency, low volatility and hygroscopicity, it is used in textile lubricants, cutting oils, dry cleaning soaps, printing inks, steam-curable inks and non-grain wood stains. In the textile industry, diethylene glycol is used as a conditioner for wool, rayon and cotton. As a solvent for dyes, it is an important auxiliary for dyeing and printing. Diethylene glycol's high hygroscopicity makes it an effective softener for tobacco, paper, synthetic sponges, glue, and casein. Diethylene glycol is particularly suitable for natural gas dehydration. A mixture of diethylene glycol and monoethanolamine removes moisture, hydrogen sulfide and carbon dioxide from natural gas. Diethylene glycol is particularly suitable for natural gas dehydration. A mixture of diethylene glycol and monoethanolamine removes moisture, hydrogen sulfide and carbon dioxide from natural gas. Diethylene glycol is particularly suitable for natural gas dehydration. A mixture of diethylene glycol and monoethanolamine removes moisture, hydrogen sulfide and carbon dioxide from natural gas. 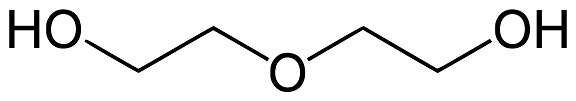 Diethylene glycol structural formula |
| use | Diethylene glycol (DEG) is a common solvent and ingredient in many commercial products. Used as a dehydrating agent in natural gas processing; as a lubricant and finishing agent for textiles; as an ingredient in brake fluids, lubricants, antifreeze formulations, wallpaper strippers and artificial fog solutions; as a solvent for printing inks and textile dyes; used in the production of certain These resins, triethylene glycol, surfactants and intermediates of diethylene glycol esters and ethers. |
| application | Diethylene glycol has many industrial uses. It is a component of antifreeze, brake fluid, cosmetics, inks and desiccants, and is used as a plasticizer. Used as antifreeze in sprinkler systems, water seals for gas storage tanks, etc. (water containing 40% diethylene glycol freezes at -18°; water containing 50% diethylene glycol freezes at -28°); used as wool Lubricating and finishing agent for , worsted, cotton, rayon, silk; used as a solvent for vat dyes; contains cork, glue, gelatin, casein and paste in its composition to prevent drying. |
| definition | ChEBI: Diethylene glycol is a hydroxy ether. |
| production method | Diethylene glycol is produced commercially as a by-product of ethylene glycol production. It can also be produced by the direct reaction of ethylene glycol and ethylene oxide. |
| general instructions | Diethylene glycol is a colorless liquid. Denser than water. Contact may cause minor irritation to skin, eyes and mucous membranes. May be slightly toxic if ingested. Used in the manufacture of other chemicals. |
| air and water reaction | Slightly soluble in water. |
| reactive profile | Diethylene glycol is incompatible with strong oxidizing agents. Diethylene glycol is also incompatible with strong bases. Diethylene glycol can react with dehydrating agents such as sulfuric acid, nitric acid, oxygen, hydrogen peroxide, perchloric acid and strong acids. It decomposes exothermically when mixed with sodium hydroxide and heated to 446° F. |
| health hazard | Ingestion of large amounts may cause degeneration of the kidneys and liver and lead to death. Liquid may cause mild skin irritation. |
| fire hazard | Diethylene glycol is flammable. |
| Flammability and explosiveness | non-flammable |
| Security overview | Moderately toxic to humans if ingested. Experimental poisoning by inhalation. Moderate toxicity by ingestion and intravenous routes. Suspected carcinogen with experimental carcinogenic, tumorigenic and teratogenic data. Irritating to eyes and human skin. Combustible when exposed to heat or flame; can react with oxidizing substances. When extinguishing fire, please use alcohol foam, water, Con, dry powder. When the mixture with sodium hydroxide is heated to 230°C, it decomposes exothermically and releases explosive hydrogen gas. When heated and decomposed, acrid and irritating fumes are released. See also glycol ethers. |
| toxicology | Diethylene glycol has similar toxicity to ethylene glycol and is apparently a central nervous system depressant. The inhalation risk is low due to low vapor pressure; however, inhalation of mists or aerosols should be avoided. Workplace vapor and aerosol levels must not exceed 50 ppm. If diethylene glycol is accidentally released, the use of a full-facepiece positive pressure respirator is recommended. Although its toxicokinetics in humans are not fully understood, its toxic properties have been confirmed by animal studies. Several human cases have been reported in the medical literature. In 1995 and 1996, several children died in Haiti after taking medicines containing diethylene glycol. Similar cases in children have been reported in other countries. A 24-year-old man developed encephalopathy and rapidly became quadriplegic after ingesting a solution containing diethylene glycol. Therefore, the toxicity of diethylene glycol is well documented. |
| Carcinogenicity | Weir et al. In a long-term study of mice of three different age levels, only one bladder tumor was found among those fed a diet containing 4% diethylene glycol. The tumor developed in a mouse that also had bladder stones. In an effort to clarify the question of tumor etiology, Weil et al. Calcium oxalate stones or glass beads were implanted into the bladders of rats. They found that bladder tumors never develop if a foreign object is present in the bladder. It was concluded that diethylene glycol, which is essentially free of ethylene glycol, is not a major carcinogen. |
| environmental fate | Diethylene glycol is mainly metabolized by alcohol dehydrogenase into toxic metabolites, HEAA and DGA. DEG can cause anion gap metabolic acidosis, cortical necrosis, leading to permanent renal failure and neurotoxicity. DGA, but not HEAA, has recently been identified as the primary nephrotoxic agent responsible for proximal tubular cell death. Neurotoxicity following DEG intoxication has only recently been described. Neurotoxicity is delayed-onset and has a sensorimotor polyneuropathy pattern of intracranial and peripheral demyelination. The exact mechanism of neurotoxicity remains unclear and, in cases described in the literature, it appears to be long-term but does show evidence of reversibility. |
| Toxicity evaluation | Diethylene glycol is miscible with water, has a low vapor pressure of 0.008 hPa at 25°C, a very low log Kow of 1.98, and a low Koc. Therefore, water is the most relevant environmental zoning. According to Mackay calculations, level I indicates the following distribution between environmental compartments: air 0.75%, water 99.25%, soil 0%, sediment 0%; confirming the correlation of pelagic systems. The substance is readily biodegradable and the very low log Kow indicates a low potential for bioaccumulation. |
| Diethylene glycol upstream and downstream product information |
Product categories
7-Hydroxybenzofuran (furan phenol) 4790-81-2
2,2-Oxydiethanol
Expandable microspheres
PP&engineering plastic lightweight functional masterbatch
N,N'-(4,4'-methylenediphenyl)bismaleimide 13676-54-5
2-Ethyl-2-(hydroxymethyl)propane-1,3-diol nonanoic acid
Xanthate
Xanthate
Didecyl phthalate 84-77-5
Dinonyl phthalate 84-76-4
Methacrylate 922-67-8
Trichloroisocyanuric acid 87-90-1
Trichloropolyamine Urate
N-undecane
4,4'-Bismaleimidediphenylmethane 13676-54-5
Isooctyl acrylate 103-11-7
Trihydroxymethylpropane
1-cyanoguanidine (dicyandiamide)
Sodium cyclamate (cyclamate) 139-05-9
Phthalic anhydride 85-44-9
Vinyl acetate 108-05-4
Diethylene glycol 111-46-6
Acetaminophen 103-90-2
Isophthalic acid 121-91-5
Didecyl phthalate 84-77-5
Methacrylate 922-67-8
Terephthalic acid (TPA) 100-21-0
Butyl acrylate 141-32-2
Methacrylate 79-41-4
Methyl methacrylate 80-62-6
Hydroxyethyl methacrylate hema 868-77-9
Dimethyl adipate 627-93-0
Dimethyl succinate 106-65-0
Dbe dibasic ester 95481-62-2
Dimethyl glutarate 1119-40-0
1,5-pentanediol 111-29-5
N-dodecane 112-40-3
Allyl glycidyl ether 106-92-3
Pyromellitic dianhydride PMDA 89-32-7
Propylene glycol methyl ether acetate PMA
Propylene glycol methyl ether PM
N-heptane 142-82-5
Tetramethylammonium hydroxide 25% 2.38% 75-59-2
Methyl acetate 79-20-9
Tetrachlorethylene 127-18-4
Trichlorethylene 79-01-6
Xylene 1330-20-7
Cyclohexanone
Absolute ethanol 64-17-5
Butyl acetate 123-86-4
Acetone 67-64-1
Butanone 78-93-3
Toluene 108-88-3
N-propyl bromide npb
Monofluorodichloroethane (HCFC-141b)
TF-B5 lead-free cleaning agent
PP treatment agent
DMP-30 Editor
Polyamide resin 63428-84-2
Isobornyl methacrylate IBOMA 7534-94-3
Dipentaerythritol hexaacrylate DPHA 29570-58-9
Stain remover oil stain remover oil stain remover oil
Film cleaner, film water, film cleaner, PCB potion
Trimethylolpropane trimethacrylate TMPTMA 3290-92-4
Trimethylolpropane triacrylate TMPTA
Scratch card ink matching thinner for screen printing
Pentaerythritol Triacrylate 3524-68-3
Methaclean
Epoxy resin 593 curing agent
Film cleaner
Isoparaffin D60 solvent oil
Different ice 98-82-8
Tripropylene glycol diacrylate TPGDA 42978-66-5
Dipropylene glycol diacrylate DPGDA
Carbon dodecyl to tetradecyl glycidyl ether 68609-97-2
HCFC-141B High Purity Cleaning Agent High Purity Sanaifu Original
1,6-Hexanediol diglycidyl ether 16096-31-4
1,4-Butanediol diglycidyl ether 2425-79-8
Flame retardant cleaning agent (non-flammable cleaning agent)
Epoxy reactive diluent 692
Neopentyl glycol diglycidyl ether 17557-23-2
Trimethylolpropane triglycidyl ether 30499-70-8
Butyl glycidyl ether
Polypropylene glycol diglycidyl ether 26142-30-3
Polyethylene glycol diglycidyl ether 39443-66-8
Paint stripper
Industrial alcohol
New solvent---a substitute for butanone
Butyl glycidyl ether (BGE) 2426-08-6
Hydrofluoroether 347 - 406-78-0
Ethyl acid ester [4]
Phenyl glycidyl ether 122-60-1
Dipropylene glycol diacrylate DPGDA
Isopropyl alcohol 67-63-0
TA-55B ink cleaning agent
Flux cleaning agent
Lead-free no-clean TF-9000 series
Traditional no-wash TF-800T series
TF-2000-3 environmentally friendly cleaning agent
GB38508-2020 cleaning agent
High pressure anti-wear hydraulic oil HM46
Halogen-free environmentally friendly cleaning agent
Circuit board cleaning agent
Synthetic high-speed electric discharge machining oil EDM-2
Stamping oil stain cleaner
Environmentally friendly flash point-free cleaning agent
Hydrocarbon ultrasonic cleaning agent
718 net washing water is
ENEOS cleaning agent Japan NSclean 100 hydrocarbon cleaning agent
High flash point hydrocarbon cleaning agent (safe and non-flammable) XHC-280 complies with VOC
Hydrocarbon cleaning agent
Lead-free washing water
Aluminum cleaning agent
Copyright © 2021. All rights reserved Thiết kế web iHappy.








