Phthalic anhydride 85-44-9
Phthalic anhydride is an organic compound with the chemical formula C6H4(CO)2O. It is an anhydride of phthalic acid. This colorless solid is an important industrial chemical, especially as a plasticizer for the mass production of plastics.
| describe |
Phthalic anhydride is an organic compound with the chemical formula C6H4(CO)2O. It is an anhydride of phthalic acid. This colorless solid is an important industrial chemical, especially as a plasticizer for the mass production of plastics. |
| chemical properties | Phthalic anhydride is a moderately flammable white solid (flaky) or clear, colorless, flowable liquid (melt). Has a spicy, suffocating smell. Very slightly soluble in H2O, soluble in ethanol, slightly soluble in ether. |
| physical properties | Colorless to pale cream crystals with a characteristic choking odour. Sensitive to moisture. The odor threshold concentration is 53 ppb (cited in Amoore and Hautala, 1983). |
| use | Phthalic anhydride is used in the manufacture of unsaturated polyesters and as a curing agent for epoxy resins. When used as a pigment, it can cause allergies in potters. |
| definition | ChEBI: Phthalic anhydride is a cyclic dicarboxylic acid anhydride, which is the anhydride of phthalic acid. It acts as an allergen. It is a cyclic dicarboxylic acid anhydride belonging to 2-benzofurans. |
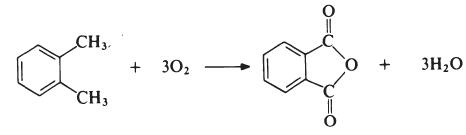
| Prepare | The most important modifying component used in the manufacture of linear unsaturated polyesters is phthalic anhydride. Acid anhydrides are generally produced by oxidation of o-xylene:
The reaction is carried out in the gas phase by passing a mixture of o-xylene and air over a catalyst, such as vanadium pentoxide supported on silica, and promoted with titanium dioxide at about 400°C. The exhaust gas was cooled, and the phthalic anhydride was collected and purified by distillation under reduced pressure. |
| synthetic references | Journal of Organic Chemistry, 25, p. 616, 1960 DOI: 10.1021/jo01074a035 Synthesis, page 616 612, 1973 Tetrahedron Letters, 20, page 612. 2301, 1979 DOI: 10.1016/S0040-4039(01)93957-7 |
| general instructions | Colorless to white glossy needle-like solid with a mild and unique odor. Moderately toxic if inhaled or ingested and irritating to skin. Melting point 64°F Flash point 305°F. Forms corrosive solution when mixed with water. Used in the manufacture of artificial resin and other materials. |
| air and water reaction | Usually reacts slowly with water to form phthalic acid and heat [Merck 11th edition. 1989]. Phthalic acid is slightly soluble in water. |
| reactive profile | Phthalic anhydride reacts exothermically with water. The reaction is sometimes slow, but can become violent when localized heating accelerates the reaction rate. Acids accelerate reactions with water. Incompatible with acids, strong oxidizing agents, alcohols, amines and bases. Exothermic nitration with fuming nitric-sulfuric acid may produce potentially explosive mixtures of phthalyl nitrates or nitrites or their nitro derivatives [Chem. & Industry 20:790. 1972] . Phthalic anhydride reacts violently with CuO at high temperatures [Park, Chang-Man, Richard J. Sheehan. Phthalic acid and other benzene polycarboxylic acids Kirk-Othmer Encyclopedia of Chemistry and Technology. John Wiley & Sons, 2005]. A mixture of phthalic anhydride and anhydrous carbon dioxide will explode violently if heated [Paper No. 5, Inst. Chemistry, London, 1940]. |
| health hazard | Solids can irritate skin and eyes, causing coughing and sneezing. Liquids can cause severe thermal burns. |
| fire hazard | Combustible material: May burn but does not readily ignite. Substances can react with water, some violently, releasing flammable, toxic or corrosive gases and runoff. When heated, the vapors may form explosive mixtures with air: explosion hazard indoors, outdoors and in sewers. Most vapors are heavier than air. They spread along the ground and collect in low or tight areas (sewers, basements, water tanks). Vapors may reach source of ignition and flash back. Contact with metal may liberate flammable hydrogen gas. Containers may explode if heated or contaminated with water. |
| pharmaceutical application | Phthalic anhydride reacts with cellulose acetate to form cellulose acetate phthalate (CAP), a common enteric coating excipient that has also been shown to have antiviral activity. Phthalic anhydride is a degradation product of CAP. |
| Contact allergens | Phthalic anhydride is used to make unsaturated polyesters and as a curing agent for epoxy resins. When used as a pigment, it may cause allergies in potters. Phthalic anhydride itself does not sensitize the resins used in nail polish phthalic anhydride/trimellitic anhydride/glycol copolymers. |
| Security overview | Poisonous if swallowed. Experimental teratogenicity. Corrosive and irritating to eyes, skin and mucous membranes. A common air pollutant. Combustible when exposed to heat or flame; can react with oxidizing substances. Moderate explosion hazard in the form of dust when exposed to flame. The production of this material caused many industrial explosions. Mixtures with copper oxide or sodium nitrite can explode when heated. Reacts violently with nitric acid + sulfuric acid above 80°C. When extinguishing fire, use carbon dioxide, dry powder chemicals. Used in plasticizers, polyester resins, alkyd resins, dyes and pharmaceuticals. See also anhydride. |
| synthesis | Phthalic anhydride is a precursor to many reagents used in organic synthesis. Important derivatives include phthalimide and its many derivatives. Chiral alcohols form half-esters (see above), and these derivatives are often resolvable because they form diastereomeric salts with chiral amines (such as strychnine). A related ring-opening reaction involves peroxides to produce useful peroxyacids: C 6 H 4 (CO) 2 O + H 2 O 2 → C 6 H 4 (CO 3 H)CO 2 H. |
| potential contact | Phthalic anhydride is used as a plasticizer; used in the production of phthalates; benzoic acid; alkyd and polyester resins; synthetic indigo; and phthalic acid; used as a plasticizer in vinyl resins. To a lesser extent, it is used in the production of alizarin, dyes, anthranilic acid; anthraquinone, diethyl phthalate; dimethyl phthalate; erythrosine, isophthalic acid; methyl Aniline, phenolphthalein, phthalamide, sulfapiperidine, and terephthalic acid. It is also used as a pesticide intermediate. |
| Shipping | UN2214 Phthalic anhydride and >.05% maleic anhydride, Hazard class: 8; Label: 8 - Corrosive material. |
| Purification method | The anhydride was distilled under reduced pressure. It was purified from the acid by extraction with hot CHCl3, filtration and evaporation. The residue is crystallized from CHCl3, CCl4 or *benzene, or sublimed. It crystallizes fractionally from the melt. Dry in vacuum at 100°C. [Saltiel J Am Chem Soc 108 2674 1986, Beilstein 17/11 V 253. ] |
| Toxicity evaluation | Phthalic anhydride modulates the release of lipid mediators and the formation of cytokines and has a sensitizing effect on the respiratory tract. In particular the local irritant effect on mucous membranes may depend on the hydrolysis of phthalic acid. |
| Incompatibility | Dust and air form explosive mixtures. Phthalic anhydride reacts exothermically with water. The reaction is sometimes slow, but can become violent when localized heating accelerates the reaction rate. Acids accelerate reactions with water. Incompatible with acids, strong oxidizing agents, alcohols, amines and bases. Converts to phthalic acid in hot water. Incompatible with oxidizing agents (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fire or explosion. Keep away from alkaline substances, strong bases, strong acids, oxygen-containing acids, and epoxides. Caustic, ammonia, amines, water. Reacts violently with copper oxide or sodium nitrite 1 heat. |
| waste disposal | Use a licensed professional waste disposal service to dispose of this material. The material is dissolved or mixed with a flammable solvent and burned in a chemical incinerator equipped with an afterburner and scrubber. All federal, state and local environmental regulations must be followed. Consult environmental regulatory agencies for guidance on acceptable disposal practices. Producers of waste containing this contaminant (≥100 kg/mo) must comply with EPA regulations for storage, transportation, handling, and waste disposal. |
| Phthalic anhydride upstream and downstream product information |